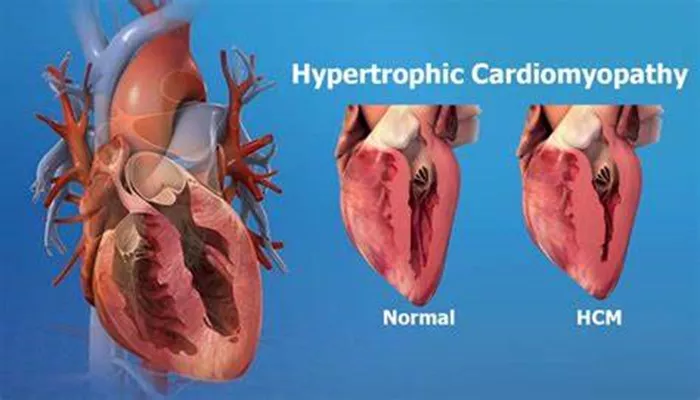
Hypertrophic cardiomyopathy (HCM) is a complex heart condition characterized by the abnormal thickening of the heart muscle, particularly affecting the left ventricle. This thickening can lead to various complications, including obstructive blood flow, diastolic dysfunction, and arrhythmias. Understanding the root causes of HCM is crucial for diagnosis, management, and potential treatment options. This article delves into the genetic and other factors contributing to HCM, providing a comprehensive overview suitable for both healthcare professionals and patients.
What Is Hypertrophic Cardiomyopathy?
Hypertrophic cardiomyopathy is primarily a genetic disorder that affects the structure and function of the heart muscle.
The condition is most often inherited in an autosomal dominant pattern, meaning that only one copy of the mutated gene from an affected parent can cause the disease in offspring. The thickening of the heart muscle can lead to several complications, including:
Obstructed blood flow: Thickened walls can block blood from leaving the heart.
Diastolic dysfunction: The heart struggles to relax and fill with blood.
Arrhythmias: Abnormal heart rhythms may develop due to structural changes in the heart.
Symptoms of HCM can vary widely among individuals, with some experiencing no symptoms at all while others may suffer from shortness of breath, chest pain, or even sudden cardiac death.
Genetic Factors Contributing to HCM
The primary root cause of hypertrophic cardiomyopathy is genetic mutations affecting proteins in the heart muscle. These mutations typically occur in genes that encode sarcomere proteins, which are essential for muscle contraction. Here are some key points regarding these genetic factors:
Familial HCM: Approximately 60% of HCM cases are familial, meaning they are inherited from parents. The most commonly affected genes include:
MYH7 (β-myosin heavy chain)
MYBPC3 (cardiac myosin-binding protein C)
Together, these two genes account for about 75% of known genetic variants associated with HCM.
Other Genetic Variants: Less commonly, mutations may occur in genes encoding thin filament proteins such as:
- Troponin T (TNNI3)
- Troponin I (TNNT2)
- Actin (ACTC1)
These mutations represent a smaller proportion of cases but are still significant.
Non-familial HCM: A notable percentage of individuals diagnosed with HCM do not have a clear genetic explanation for their condition. This non-familial form may arise from environmental factors or other unknown mechanisms.
Mechanisms Leading to Hypertrophic Cardiomyopathy
Several mechanisms contribute to the development and progression of hypertrophic cardiomyopathy:
Dynamic Left Ventricular Outflow Tract Obstruction (LVOTO):
In many patients, the thickened septum can obstruct blood flow from the left ventricle during contraction. This obstruction can occur at rest or during physical exertion.
A significant pressure gradient (≥30 mm Hg) across the outflow tract is clinically relevant and may necessitate surgical intervention if symptomatic.
Diastolic Dysfunction:
The thickened heart muscle becomes stiff, impairing its ability to relax and fill adequately with blood during diastole.
This can lead to increased pressures in the heart and lungs, causing symptoms like shortness of breath.
Myocardial Ischemia:
The thickened muscle may require more oxygen than it receives due to reduced coronary artery perfusion.
This mismatch can lead to angina or chest pain during physical activity.
Arrhythmias:
Structural changes in the heart muscle can disrupt normal electrical conduction pathways, leading to arrhythmias.
Patients with HCM are at increased risk for atrial fibrillation and other potentially life-threatening arrhythmias.
Mitral Valve Abnormalities:
The mitral valve may not function properly due to changes in geometry caused by hypertrophy.
This can lead to mitral regurgitation, where blood leaks backward into the left atrium during contraction.
Risk Factors for Developing HCM
While genetic predisposition plays a significant role in developing hypertrophic cardiomyopathy, several other factors may influence its onset and severity:
Family History: A family history of HCM significantly increases an individual’s risk. First-degree relatives of affected individuals should undergo screening tests.
Age and Gender: Symptoms often present during adolescence or young adulthood; however, they can occur at any age.
There is no strong gender predilection in most cases.
Athletic Activity: Young athletes may experience sudden cardiac events due to undiagnosed HCM, highlighting the importance of screening in this population.
Diagnosis of Hypertrophic Cardiomyopathy
Diagnosing hypertrophic cardiomyopathy typically involves a combination of clinical evaluation and diagnostic testing:
Medical History and Physical Examination: A thorough history focusing on symptoms and family history is essential.
Electrocardiogram (ECG): This test helps identify abnormal heart rhythms or signs of left ventricular hypertrophy.
Echocardiogram: An ultrasound of the heart provides detailed images showing wall thickness and structural abnormalities.
Genetic Testing: If familial HCM is suspected, genetic testing can confirm mutations associated with the disease.
Management And Treatment Options
While there is currently no cure for hypertrophic cardiomyopathy, several management strategies aim to alleviate symptoms and prevent complications:
Medications: Beta-blockers or calcium channel blockers are often prescribed to reduce heart rate and improve symptoms related to obstruction.
Lifestyle Modifications: Patients are advised to avoid strenuous exercise that could provoke symptoms or increase risk.
Surgical Interventions: In cases of significant obstruction or severe symptoms not responding to medication, surgical options such as myectomy (removal of excess heart muscle) may be considered.
Regular Monitoring: Continuous follow-up with a healthcare provider is crucial for managing this condition effectively.
Conclusion
Hypertrophic cardiomyopathy represents a significant health concern due to its genetic basis and potential for severe complications. Understanding its root causes—primarily genetic mutations affecting sarcomere proteins—enables better diagnosis and management strategies. Ongoing research continues to explore additional mechanisms contributing to this complex condition, aiming for improved patient outcomes through tailored therapeutic approaches.
Related topics:
- Can Covid Cause Dilated Cardiomyopathy?
- What Are The Odds of Getting Myocarditis from Covid Booster?
- Can You Fully Recover From Dilated Cardiomyopathy?