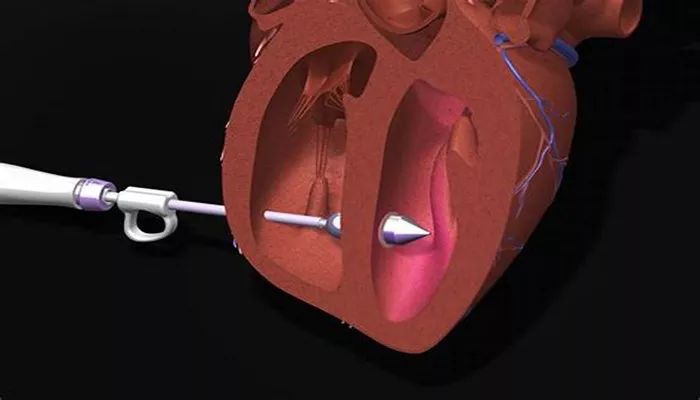
Abbott Laboratories has launched a new leadless pacemaker in India, aimed at treating patients with slow heart rhythms.
The device, known as the AVEIR VR single-chamber ventricular leadless pacemaker, has received approval from the Central Drugs Standard Control Organisation (CDSCO) in India, as well as from the U.S. Food and Drug Administration (USFDA).
Ajay Singh Chauhan, General Manager for Abbott’s Cardiac Rhythm Management division in India and Southeast Asia, highlighted that the AVEIR VR pacemaker is designed to simplify both the implantation and retrieval processes for physicians. This innovation aims to enhance patient care by addressing the needs of those experiencing bradycardia, a condition characterized by a slower-than-normal heart rate. Pacemakers deliver electrical pulses to help regulate heartbeats effectively.
Balbir Singh, Chairman of Cardiology at Max Superspeciality Hospital, noted that leadless pacemakers mitigate complications often associated with traditional devices. Unlike conventional pacemakers, which require a surgical incision in the chest and the use of insulated wires called cardiac leads, leadless pacemakers eliminate the need for these components. This design reduces the risk of infection and other complications.
Vanita Arora, Clinical Lead in Electrophysiology at Apollo Hospitals Delhi, emphasized the importance of retrievability in certain cases where a patient’s treatment needs may evolve over time. The ability to retrieve the device can be crucial for future adjustments.
The introduction of Abbott’s AVEIR VR pacemaker marks a significant advancement in cardiac care technology. It not only provides an effective solution for managing slow heart rhythms but also represents a shift towards less invasive procedures that prioritize patient comfort and safety.
Related topics: