Innoventric, a startup based in Ness Ziona, Israel, is making strides in the treatment of tricuspid valve leaks, a common heart condition that can lead to serious health issues. The company has recently secured $28.5 million in funding to support its innovative device, Trillium, which is currently undergoing clinical trials.
The Trillium device offers a groundbreaking approach to treating tricuspid regurgitation, a condition where the tricuspid valve fails to close properly, allowing blood to flow backward into the right atrium. This condition can lead to complications such as heart enlargement and increased workload on the heart, potentially resulting in severe health risks if left untreated.
A Quick And Less Invasive Procedure
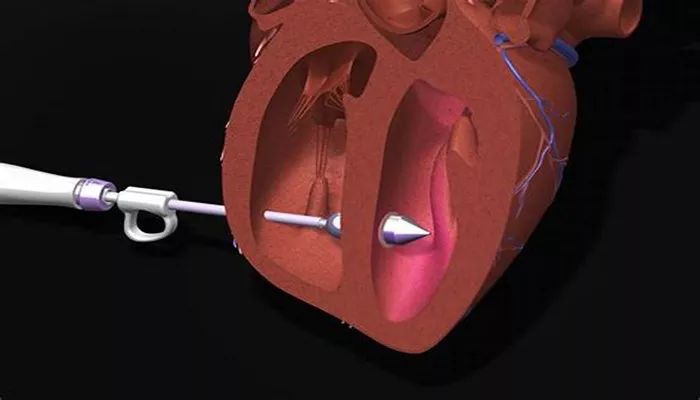
Unlike traditional methods that involve complex surgeries to replace the faulty valve, Innoventric’s Trillium can be implanted in a quick 10-minute procedure. This procedure is performed under conscious sedation and does not require general anesthesia or echocardiography.
The device contains several one-way valves and is placed in the large veins that carry deoxygenated blood, specifically the superior and inferior vena cava. This innovative approach avoids the complications associated with directly altering the tricuspid valve’s anatomy.
Clinical Trials And Regulatory Approvals
Innoventric has successfully completed initial clinical trials in Europe and is now treating patients in the United States after receiving clearance from the U.S. Food and Drug Administration (FDA) for an early feasibility study. The recent funding will help advance these clinical trials and expand regulatory approvals in both the U.S. and Europe.
Approximately 4% of individuals over the age of 75 experience moderate tricuspid regurgitation. If untreated, this condition can lead to significant heart complications.
How Tricuspid Regurgitation Affects Patients
In a healthy heart, the tricuspid valve closes tightly when the right ventricle contracts, preventing blood from leaking back into the right atrium. However, with tricuspid regurgitation (TR), this valve does not seal completely, causing blood to flow backward. Over time, this can cause the right atrium to enlarge and reduce blood flow to the lungs, forcing the heart to work harder. If left untreated, TR can be fatal.
Advantages of Innoventric’s Treatment
The Trillium device reduces patient risk by eliminating the need for surgical valve replacement and general anesthesia. This minimally invasive approach not only shortens recovery times but also boasts a higher success rate compared to traditional methods. Moreover, it is suitable for a broader range of patients, including those who may not qualify for other tricuspid procedures.
Amir Danino, CEO of Innoventric, emphasized the company’s mission: “Our goal is to transform care for tricuspid regurgitation with minimally invasive therapies that significantly enhance patient outcomes.” He added that the recent investment reflects both investor confidence and the substantial potential of their approach to treating TR.
Funding Details
The Series B funding round was led by RA Capital Management from the U.S., with participation from the European Innovation Council (EIC) Fund and returning investors such as BRM Group, JG Private Equity, and Mivtach Shamir Holdings.
With this new investment, Innoventric’s total funding since its inception in 2017 has reached $41 million.
Innoventric’s advancements in treating tricuspid regurgitation through its Trillium device could significantly improve outcomes for patients suffering from this common yet serious heart condition. As clinical trials progress and regulatory approvals expand, Innoventric aims to make this innovative treatment widely available to those in need.
Related topics:
- HCA Florida Gulf Coast Hospital Honored for Excellence in Coronary Care
- Global Market for Structural Heart Devices Expected to Reach $22.16 Billion by 2029
- UVA Health Introduces Innovative Method for Heart Valve Replacement