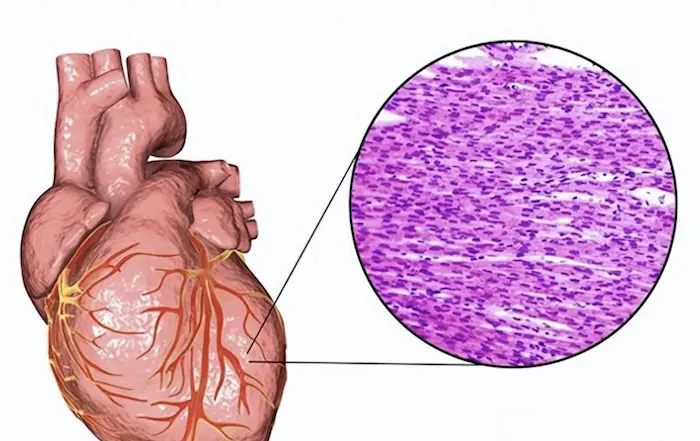
Giant cell myocarditis (GCM) is a rare and severe form of myocarditis characterized by the presence of multinucleated giant cells in the myocardial tissue. It is often associated with significant cardiac dysfunction, arrhythmias, and a high risk of sudden cardiac death. Given the serious implications of this condition, understanding its etiology, including the potential hereditary factors, is crucial for both healthcare professionals and patients. This article explores the question of whether giant cell myocarditis is hereditary, examining genetic predispositions, environmental factors, and the interplay between genetics and immune responses.
Understanding Giant Cell Myocarditis
Definition and Pathophysiology
Giant cell myocarditis is an inflammatory disease of the heart muscle that is often classified as autoimmune in nature. The pathophysiology involves.
Immune Dysregulation: The immune system mistakenly attacks the heart muscle, leading to inflammation and damage. This may be triggered by infections, autoimmune diseases, or environmental factors.
Formation of Giant Cells: The hallmark of GCM is the presence of multinucleated giant cells, which are formed by the fusion of macrophages in response to chronic inflammation.
Myocardial Injury: The inflammatory process leads to myocyte necrosis, fibrosis, and impaired cardiac function. This can result in symptoms of heart failure, arrhythmias, and other serious complications.
Epidemiology of Giant Cell Myocarditis
Giant cell myocarditis is considered a rare condition, with an estimated incidence of 1-2 cases per million people per year. It predominantly affects young to middle-aged adults, typically between the ages of 20 and 50, with no significant gender predisposition. The condition can occur in isolation or in association with other autoimmune diseases, such as systemic lupus erythematosus or rheumatoid arthritis.
Genetic Factors in Giant Cell Myocarditis
Hereditary Components of Autoimmune Diseases
While giant cell myocarditis itself is not classified as a hereditary disease, there is evidence suggesting that genetic factors may play a role in its development, particularly in the context of autoimmune diseases. Many autoimmune disorders have a known genetic component, and individuals with a family history of autoimmune diseases may be at an increased risk for developing GCM.
Genetic Susceptibility: Certain genetic polymorphisms have been associated with an increased risk of autoimmune diseases. For example, variations in genes related to immune regulation, such as those encoding major histocompatibility complex (MHC) molecules, can influence susceptibility to autoimmune conditions.
Familial Patterns: Some studies have reported familial clustering of autoimmune diseases, suggesting a potential genetic predisposition. While specific studies on GCM are limited, the observation that other autoimmune diseases can run in families raises questions about the potential hereditary nature of GCM.
Specific Genetic Associations
Research into the genetic basis of giant cell myocarditis is still in its early stages, and specific genetic markers associated with GCM have not been definitively identified. However, some studies have explored the potential involvement of certain genes.
HLA Genes: Human leukocyte antigen (HLA) genes play a crucial role in the immune response. Variants in HLA genes have been implicated in various autoimmune diseases, and their role in GCM may warrant further investigation.
Cytokine Genes: Genes involved in cytokine production and regulation may also be relevant. Dysregulation of cytokines can contribute to inflammation and autoimmune processes, potentially influencing the development of GCM.
Other Immune-Related Genes: Variants in genes associated with immune cell function, such as those encoding for T cell receptors or signaling pathways, may also be implicated in the pathogenesis of GCM.
Environmental Triggers and Their Interaction with Genetics
While genetic factors may contribute to the risk of developing giant cell myocarditis, environmental triggers also play a significant role. The interaction between genetic predisposition and environmental factors is crucial in understanding the etiology of autoimmune diseases.
Infections: Viral infections, particularly those caused by enteroviruses, adenoviruses, and parvovirus B19, have been implicated in the development of myocarditis. In genetically predisposed individuals, an infection may trigger an autoimmune response leading to GCM.
Autoimmune Diseases: The presence of other autoimmune conditions can increase the risk of developing GCM. For example, patients with systemic lupus erythematosus or rheumatoid arthritis may have a higher likelihood of experiencing GCM due to the shared underlying immune dysregulation.
Environmental Exposures: Factors such as toxins, drugs, and other environmental exposures may also trigger or exacerbate autoimmune responses in susceptible individuals.
Lifestyle Factors: Lifestyle factors, including diet, exercise, and stress, may influence immune function and inflammation, potentially affecting the risk of developing GCM in genetically predisposed individuals.
Clinical Presentation of Giant Cell Myocarditis
Symptoms and Signs
Giant cell myocarditis can present with a variety of symptoms, which may vary in severity and duration. Common clinical manifestations include.
Chest Pain: Patients may experience chest pain resembling that of myocardial infarction.
Heart Failure Symptoms: Symptoms such as dyspnea, fatigue, and peripheral edema may occur due to impaired cardiac function.
Arrhythmias: Patients may present with palpitations, syncope, or sudden cardiac arrest due to conduction disturbances.
Systemic Symptoms: Fever, malaise, and other systemic symptoms may be present, reflecting the underlying inflammatory process.
Disease Progression
Giant cell myocarditis can progress rapidly, often leading to severe heart failure or sudden cardiac death within weeks to months of symptom onset. Early recognition and treatment are critical to improving outcomes.
Diagnosis of Giant Cell Myocarditis
Diagnosing giant cell myocarditis involves a multi-step approach that includes clinical evaluation, laboratory tests, imaging studies, and histopathological examination. Each component plays a crucial role in establishing the diagnosis and ruling out other potential causes of myocarditis.
Clinical Evaluation
A thorough medical history and physical examination are essential in the diagnostic process. Key components include.
History of Symptoms: Understanding when symptoms began and their progression can help assess the severity of the disease.
Previous Medical History: A history of autoimmune diseases, viral infections, or recent vaccinations can provide important clues.
Physical Examination: Vital signs, cardiac exam findings, and signs of heart failure should be assessed.
Electrocardiogram (ECG)
An ECG may reveal various abnormalities, including ST-segment changes, arrhythmias, or conduction abnormalities, which can provide additional diagnostic information.
Laboratory Tests
Laboratory tests may include cardiac biomarkers (e.g., troponin), inflammatory markers (e.g., C-reactive protein), and autoimmune panels to support the diagnosis and rule out other conditions.
Imaging Studies
Echocardiography and cardiac magnetic resonance imaging (MRI) are essential for assessing cardiac function and identifying structural abnormalities. Cardiac MRI, in particular, can provide detailed information about myocardial inflammation and scarring.
Endomyocardial Biopsy
The definitive diagnosis of giant cell myocarditis is made through an endomyocardial biopsy, which involves obtaining a small sample of myocardial tissue for histopathological examination. The presence of multinucleated giant cells confirms the diagnosis.
Treatment of Giant Cell Myocarditis
The treatment of giant cell myocarditis is multifaceted and aims to reduce inflammation, manage heart failure symptoms, and prevent complications. The following sections outline the primary treatment modalities available.
Immunosuppressive Therapy
Given the autoimmune nature of giant cell myocarditis, immunosuppressive therapy is the cornerstone of treatment. The main options include.
Corticosteroids
Mechanism of Action: Corticosteroids, such as prednisone, are anti-inflammatory agents that reduce immune-mediated damage to the myocardium.
Dosage and Administration: The initial dose typically ranges from 1 to 2 mg/kg/day, tapering down based on clinical response and side effects. Higher doses may be required in severe cases.
Monitoring: Patients receiving corticosteroids should be monitored for potential side effects, including infections, hyperglycemia, and gastrointestinal complications.
Additional Immunosuppressive Agents
In cases where corticosteroids alone are insufficient or in patients with severe disease, additional immunosuppressive agents may be considered.
Azathioprine: This medication inhibits purine synthesis and can help reduce immune-mediated inflammation. It is often used in combination with corticosteroids.
Mycophenolate Mofetil: This agent inhibits lymphocyte proliferation and is another option for patients who do not respond adequately to corticosteroids.
Cyclophosphamide: In refractory cases, cyclophosphamide may be used as a more potent immunosuppressive agent.
Rituximab: This monoclonal antibody targets CD20 on B cells and may be considered in cases with a significant autoimmune component.
Heart Failure Management
Patients with giant cell myocarditis often present with heart failure symptoms that require appropriate management. The following strategies are commonly employed.
Diuretics
Role: Diuretics are used to manage fluid overload and relieve symptoms of congestion. They help reduce preload and improve symptoms such as dyspnea and peripheral edema.
Types: Loop diuretics (e.g., furosemide) are commonly used due to their potency and rapid onset of action.
Angiotensin-Converting Enzyme (ACE) Inhibitors
Role: ACE inhibitors help reduce afterload and improve cardiac output, making them beneficial in heart failure management.
Examples: Medications such as lisinopril or ramipril are commonly prescribed.
Beta-Blockers
Role: Beta-blockers can improve symptoms of heart failure, reduce heart rate, and decrease myocardial oxygen demand.
Caution: While beta-blockers are beneficial in chronic heart failure, they should be used cautiously in acute settings where hypotension may be a concern.
Aldosterone Antagonists
Role: Aldosterone antagonists, such as spironolactone, can help manage fluid retention and provide additional mortality benefits in heart failure patients.
Arrhythmia Management
Arrhythmias are common in giant cell myocarditis and can lead to significant morbidity. Management strategies include.
Antiarrhythmic Medications
Indications: Antiarrhythmic drugs may be prescribed to manage atrial fibrillation, ventricular tachycardia, or other arrhythmias.
Examples: Medications such as amiodarone or sotalol may be used based on the specific arrhythmia and patient characteristics.
Implantable Cardioverter-Defibrillators (ICDs)
Indications: In patients with a high risk of sudden cardiac death or significant ventricular arrhythmias, an ICD may be indicated.
Role: ICDs provide lifesaving therapy by detecting and treating life-threatening arrhythmias.
Advanced Therapies
For patients with severe heart failure or those who do not respond to medical therapy, advanced treatment options may be considered.
Mechanical Circulatory Support
Indications: Devices such as ventricular assist devices (VADs) may be used to support cardiac function in patients awaiting heart transplantation or those with refractory heart failure.
Role: VADs can help maintain adequate circulation and improve quality of life while waiting for definitive treatment.
Heart Transplantation
Indications: Heart transplantation may be considered for patients with refractory heart failure or severe cardiac dysfunction who do not respond to medical therapy.
Challenges: The decision to proceed with transplantation involves careful consideration of the patient’s overall health, the presence of comorbidities, and the availability of donor organs.
Supportive Care
In addition to specific treatment strategies, supportive care plays a crucial role in managing patients with giant cell myocarditis.
Monitoring: Regular follow-up with echocardiography and cardiac MRI is essential to assess disease progression and response to treatment.
Lifestyle Modifications: Patients should be encouraged to adopt heart-healthy lifestyle changes, including a low-sodium diet, regular exercise, and smoking cessation.
Psychosocial Support: Providing emotional support and counseling can help patients cope with the psychological impact of living with a chronic and potentially life-threatening condition.
Conclusion
While giant cell myocarditis is not classified as a hereditary disease, genetic factors may play a role in its development, particularly in the context of autoimmune diseases. The interplay between genetic predisposition and environmental triggers is crucial in understanding the etiology of GCM. Further research is needed to elucidate specific genetic markers associated with giant cell myocarditis and to explore the mechanisms by which genetic factors may influence the risk of developing this severe condition.
By understanding the potential hereditary components and the broader context of autoimmune diseases, healthcare professionals can better identify at-risk individuals and provide timely interventions. As we continue to learn more about giant cell myocarditis, there is hope for improved diagnostic and therapeutic strategies that can enhance patient outcomes and quality of life.
Related Topics: