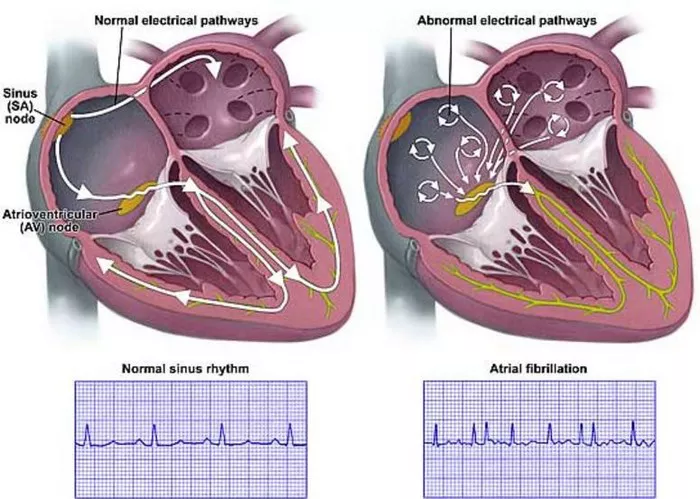
Atrial fibrillation (AFib) stands as one of the most prevalent cardiac arrhythmias worldwide, affecting millions of individuals and presenting significant challenges in terms of management and treatment. Despite advancements in medical science, the exact etiology of AFib remains multifactorial and not fully elucidated. In this comprehensive article, we delve into the intricate web of factors contributing to the development and perpetuation of AFib, ranging from genetic predispositions to lifestyle influences and comorbidities. By gaining a deeper understanding of the underlying causes of AFib, healthcare professionals can better tailor treatment strategies and preventive measures, ultimately improving patient outcomes.
Unraveling the Mystery of AFib
Atrial fibrillation, characterized by irregular and often rapid heartbeats originating from the atria, significantly increases the risk of stroke, heart failure, and other cardiovascular complications. The pathophysiology of AFib involves complex interactions between electrical, structural, and functional components of the heart. While advancements in research have shed light on various aspects of AFib, a comprehensive understanding of its etiology remains elusive. This article aims to explore the multifaceted nature of AFib causation, integrating findings from genetics, physiology, epidemiology, and clinical observations.
Genetics: Unraveling the Genetic Predisposition
AFib Genetic Basis and Inheritance Patterns
Genetic predisposition plays a crucial role in the development of AFib, with familial aggregation observed in a significant proportion of cases. Genome-wide association studies (GWAS) have identified numerous genetic loci associated with AFib susceptibility, implicating genes involved in cardiac ion channels, structural proteins, and signaling pathways. The inheritance patterns of AFib exhibit both familial clustering and sporadic occurrences, suggesting a complex interplay between genetic susceptibility and environmental factors.
Channelopathies and Structural Abnormalities
Mutations in genes encoding cardiac ion channels, such as KCNQ1, KCNH2, and SCN5A, contribute to the pathogenesis of familial forms of AFib. These channelopathies disrupt the normal electrical activity of the heart, leading to arrhythmogenic changes in atrial repolarization and refractoriness. Additionally, structural abnormalities in the atria, including fibrosis, hypertrophy, and dilation, increase the vulnerability to AFib by altering conduction properties and promoting reentrant circuits.
Hemodynamic Factors: Impact of Pressure and Volume Overload
Hypertension: The Silent Precursor
Chronic hypertension represents a major risk factor for AFib, exerting hemodynamic stress on the atria and promoting electrical remodeling. Elevated systemic blood pressure contributes to atrial stretch and dilation, fostering atrial fibrosis and electrical instability. Moreover, hypertensive heart disease predisposes individuals to left atrial enlargement and diastolic dysfunction, creating a substrate conducive to AFib initiation and perpetuation.
Valvular Heart Disease: Altered Hemodynamics and Endothelial Dysfunction
Valvular heart disease, particularly mitral valve disorders, poses a significant risk for AFib development. Mitral valve regurgitation and stenosis disrupt normal blood flow patterns, leading to atrial remodeling and endothelial dysfunction. The resulting turbulence and shear stress promote atrial inflammation and fibrosis, facilitating the onset of AFib. Additionally, prosthetic heart valves and infective endocarditis further compound the proarrhythmic milieu within the atria.
Inflammation and Oxidative Stress: Fueling Atrial Remodeling
Inflammatory Pathways in AFib Pathogenesis
Chronic inflammation represents a common denominator in the pathophysiology of AFib, contributing to atrial structural remodeling and electrical instability. Inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha), modulate cardiac fibroblast activity and promote collagen deposition within the atrial myocardium. Activation of the innate immune system triggers a cascade of events culminating in atrial fibrosis and conduction abnormalities, predisposing to AFib onset.
Oxidative Stress and Mitochondrial Dysfunction
Increased oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and antioxidant defenses, has emerged as a key determinant of AFib susceptibility. Dysfunctional mitochondria within cardiomyocytes generate excessive ROS, triggering cellular oxidative damage and apoptosis. Moreover, oxidative modifications of ion channels and calcium-handling proteins disrupt normal cardiac electrophysiology, fostering arrhythmogenic substrate formation.
Neurohormonal Dysregulation: Sympathetic Overdrive and Autonomic Imbalance
Sympathovagal Imbalance in AFib Pathophysiology
The autonomic nervous system exerts profound influences on cardiac electrophysiology and rhythm control, with sympathetic overactivity and parasympathetic withdrawal implicated in AFib initiation and maintenance. Baroreceptor reflex dysfunction and catecholamine excess enhance atrial automaticity and trigger ectopic beats, precipitating AFib episodes. Moreover, neural remodeling within the atria alters neurotransmitter release and receptor expression, perpetuating the arrhythmogenic substrate.
Renin-Angiotensin-Aldosterone System Activation
Activation of the renin-angiotensin-aldosterone system (RAAS) contributes to atrial fibrosis and electrical remodeling, fueling the progression of AFib. Angiotensin II, a potent vasoconstrictor, promotes oxidative stress and inflammation within the atrial myocardium, fostering fibroblast activation and collagen synthesis. Additionally, aldosterone-mediated sodium retention and potassium loss disrupt cellular ion homeostasis, predisposing to atrial arrhythmias.
Lifestyle Factors: The Intersection of Behavior and Biology
Obesity and Metabolic Syndrome: Adipose Tissue Dysfunction
Obesity and metabolic syndrome represent significant risk factors for AFib, exerting systemic and atrial-specific effects on cardiovascular health. Adipose tissue dysfunction, characterized by adipokine dysregulation and chronic low-grade inflammation, contributes to atrial fibrosis and conduction abnormalities. Furthermore, insulin resistance and dyslipidemia promote atrial electrical remodeling, fostering arrhythmogenic substrate formation.
Physical Inactivity and Sedentary Behavior
Sedentary lifestyle and physical inactivity pose independent risks for AFib development, underscoring the importance of regular exercise in cardiovascular health maintenance. Prolonged sitting and reduced physical exertion disrupt autonomic balance and impair cardiac function, predisposing individuals to atrial ectopy and arrhythmia vulnerability. Conversely, structured exercise training exerts beneficial effects on atrial electrophysiology and remodeling, mitigating AFib risk.
Conclusion
Atrial fibrillation represents a complex interplay of genetic, hemodynamic, inflammatory, neurohormonal, and lifestyle factors, with each contributing to the initiation and perpetuation of arrhythmia. A comprehensive understanding of AFib etiology is essential for risk stratification, personalized therapy selection, and targeted preventive interventions. Future research endeavors should focus on elucidating the intricate molecular mechanisms underlying AFib pathophysiology, paving the way for innovative therapeutic strategies and precision medicine approaches. By unraveling the mysteries of AFib causation, healthcare providers can optimize patient care and improve long-term outcomes in this challenging cardiac condition.