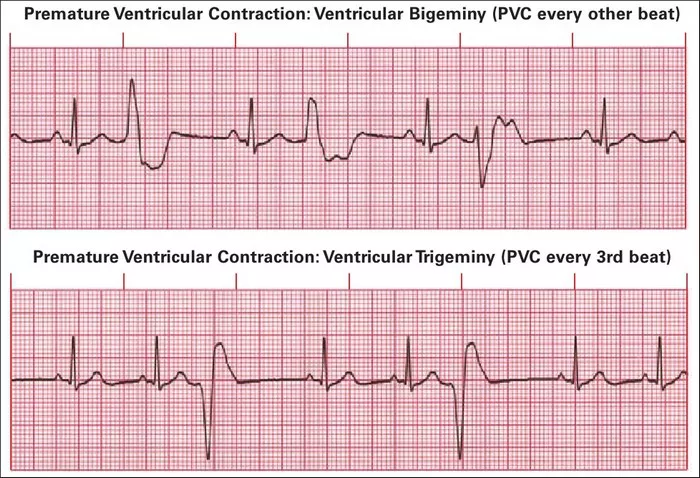
Premature Ventricular Contractions (PVCs) are ectopic heartbeats originating in the ventricles, occurring earlier than the next expected normal heartbeat. They are a common cardiac arrhythmia often detected incidentally during routine clinical evaluations or while monitoring for other cardiac conditions. PVCs can manifest at rest, during physical activity, or even during sleep, but understanding the underlying causes of PVCs at rest poses a particular challenge to clinicians and researchers.
Premature Ventricular Contractions (PVCs) are a frequent clinical finding, often perceived as benign, but they can cause significant distress and anxiety in patients, particularly when they occur at rest. While PVCs are commonly observed in individuals with structurally normal hearts, they can also signify underlying cardiac pathology. The occurrence of PVCs at rest presents a unique puzzle to cardiac specialists, requiring a comprehensive evaluation of potential triggers and contributing factors.
Anatomy and Physiology of Premature Ventricular Contractions
To comprehend the mechanisms underlying PVCs at rest, it’s imperative to understand the normal physiology of cardiac conduction. The heart’s rhythm is orchestrated by the coordinated activation and relaxation of cardiac muscle cells, initiated by electrical impulses generated in the sinoatrial node (SA node). These impulses travel through the atria, stimulating atrial contraction, before reaching the atrioventricular (AV) node and subsequently conducting to the ventricles, prompting ventricular contraction.
PVCs occur when an ectopic focus within the ventricles generates an impulse, bypassing the normal conduction pathway. This premature impulse triggers a premature ventricular contraction before the next expected impulse from the SA node. PVCs are classified based on their morphology, timing, and frequency, with complex subtypes indicative of various underlying mechanisms.
Risk Factors for PVCs at Rest
Understanding the risk factors associated with PVCs at rest is crucial for risk stratification and management. While PVCs can occur in individuals without underlying cardiac conditions, certain predisposing factors increase their likelihood and severity.
1. Structural Heart Disease: Individuals with structural abnormalities in the heart, such as myocardial infarction, cardiomyopathy, or valvular heart disease, are at an increased risk of developing PVCs at rest. These structural changes can create areas of abnormal electrical activity, fostering the initiation and propagation of ectopic beats.
2. Electrolyte Imbalance: Disturbances in electrolyte levels, particularly potassium, magnesium, and calcium, can disrupt the normal electrical activity of the heart and predispose individuals to PVCs. Hypokalemia, in particular, is strongly associated with PVC occurrence and severity.
3. Sympathetic Overstimulation: Excessive sympathetic nervous system activity, often triggered by stress, anxiety, or stimulant use, can precipitate PVCs at rest. Sympathetic activation alters cardiac autonomic tone, leading to increased automaticity and triggered activity in cardiac cells.
4. Medication and Substance Use: Certain medications, such as beta-blockers, antiarrhythmics, and psychotropic drugs, can either directly induce PVCs or exacerbate existing arrhythmias. Similarly, substances like caffeine, nicotine, and illicit drugs can trigger PVCs through their effects on cardiac electrophysiology.
5. Sleep Disorders: Sleep disturbances, including obstructive sleep apnea and insomnia, have been linked to an increased prevalence of PVCs at rest. The interplay between sympathetic activation, hypoxia, and altered autonomic regulation during sleep contributes to arrhythmogenesis.
Pathophysiology of PVCs at Rest
The pathophysiology of PVCs at rest is multifactorial, involving a complex interplay of genetic, structural, and environmental factors. While the precise mechanisms underlying PVC initiation and maintenance remain incompletely understood, several key processes contribute to their occurrence.
1. Triggered Activity: PVCs can arise from triggered activity, wherein abnormal depolarizations occur due to afterdepolarizations triggered by preceding action potentials. Early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs) are two types of triggered activity implicated in PVC genesis, often potentiated by electrolyte imbalances and sympathetic stimulation.
2. Reentry Circuits: Reentry, a common mechanism underlying various cardiac arrhythmias, occurs when a propagating impulse re-excites previously depolarized tissue, perpetuating a self-sustaining circuit. Reentry circuits can form within the ventricular myocardium, leading to repetitive PVCs at rest, particularly in the setting of scarred or fibrotic tissue.
3. Automaticity: Abnormal automaticity, defined as the ability of cardiac cells to spontaneously depolarize, plays a significant role in PVC initiation. Enhanced automaticity in ectopic foci within the ventricles can trigger PVCs independent of the normal cardiac conduction system, contributing to their occurrence at rest.
4. Structural Remodeling: Structural alterations in the myocardium, such as fibrosis, hypertrophy, or ischemic injury, create a substrate conducive to arrhythmogenesis. These changes disrupt normal conduction pathways, promote reentry circuits, and facilitate ectopic beat generation, predisposing individuals to PVCs at rest.
Diagnostic Evaluation of PVCs at Rest
Accurate diagnosis and risk stratification of PVCs at rest are essential for guiding management and prognostication. The diagnostic approach typically involves a combination of clinical assessment, electrocardiographic monitoring, and adjunctive testing modalities.
1. Electrocardiography (ECG): Standard 12-lead ECG remains the cornerstone for PVC detection and characterization. PVCs typically manifest as premature QRS complexes with abnormal morphologies, such as wide QRS duration (>120 ms), bizarre T-wave inversions, and absence of preceding P waves.
2. Ambulatory Holter Monitoring: Ambulatory ECG monitoring over 24 to 48 hours allows for the detection of PVC frequency, patterns, and associated symptoms during daily activities and rest. Holter monitoring is particularly useful for assessing PVC burden and correlating arrhythmia occurrence with symptoms.
3. Event Recorders: Event recorders provide on-demand ECG recording upon symptomatic activation by the patient, facilitating the capture of infrequent or paroxysmal PVCs at rest. These devices are valuable for correlating arrhythmia episodes with symptoms and guiding subsequent management.
4. Cardiac Imaging: Structural and functional cardiac imaging modalities, including echocardiography, cardiac magnetic resonance imaging (MRI), and coronary angiography, are employed to evaluate underlying structural heart disease and assess for myocardial ischemia or scar formation.
5. Electrophysiological Studies (EPS): Invasive EPS may be indicated in select cases, particularly when non-invasive evaluations fail to elucidate the underlying mechanism or when ablative therapy is considered. EPS allows for precise mapping of arrhythmogenic foci and assessment of inducibility of ventricular arrhythmias.
Management Strategies for PVCs at Rest
The management of PVCs at rest is guided by the underlying etiology, symptom severity, and associated comorbidities. While many individuals with isolated PVCs have an excellent prognosis, certain high-risk populations may require more aggressive intervention to mitigate adverse outcomes.
1. Lifestyle Modification: Lifestyle modifications aimed at reducing sympathetic tone and minimizing arrhythmia triggers are often recommended as first-line therapy. These may include stress reduction techniques, avoidance of stimulants like caffeine and nicotine, regular exercise, and optimizing sleep hygiene.
2. Medication Therapy: Pharmacological management of PVCs may be warranted in symptomatic individuals or those at high risk of arrhythmia-related complications. Antiarrhythmic medications, such as beta-blockers, calcium channel blockers, or sodium channel blockers, are commonly used to suppress ectopic activity and reduce PVC burden.
3. Catheter Ablation: Catheter ablation, a minimally invasive procedure targeting arrhythmogenic foci within the heart, may be considered in refractory cases or when PVCs arise from specific identifiable substrates. Ablation aims to eliminate or modify the ectopic focus, thereby interrupting the arrhythmia circuit and restoring normal cardiac rhythm.
4. Implantable Devices: In individuals with severe symptomatic PVCs or those at high risk of sudden cardiac death due to underlying structural heart disease, implantable cardiac devices such as implantable cardioverter-defibrillators (ICDs) may be indicated for primary or secondary prevention.
5. Treatment of Underlying Conditions: Addressing underlying cardiac conditions contributing to PVC occurrence, such as myocardial ischemia, heart failure, or electrolyte imbalances, is paramount in managing PVCs at rest. Optimal management of comorbidities can reduce arrhythmia burden and improve clinical outcomes.
Prognosis and Follow-up
The prognosis of PVCs at rest varies depending on the underlying etiology, PVC burden, and associated comorbidities. In individuals with isolated PVCs and structurally normal hearts, the prognosis is generally favorable, with a low risk of adverse cardiac events. However, in the presence of significant structural heart disease or high PVC burden, the risk of arrhythmia-related complications, including heart failure exacerbation, ventricular dysfunction, and sudden cardiac death, may be increased.
Regular follow-up and monitoring are essential to assess treatment response, evaluate arrhythmia burden, and detect any progression of underlying cardiac pathology. Close collaboration between primary care providers, cardiologists, and electrophysiologists is crucial in optimizing management strategies and ensuring comprehensive cardiac care.
Conclusion
Premature Ventricular Contractions (PVCs) at rest represent a common yet enigmatic cardiac arrhythmia, posing diagnostic and therapeutic challenges to clinicians. While PVCs are often benign in individuals with structurally normal hearts, their occurrence at rest may signify underlying cardiac pathology, electrolyte disturbances, or autonomic dysregulation. A thorough understanding of the risk factors, pathophysiology, and diagnostic evaluation of PVCs is essential for guiding appropriate management strategies and optimizing clinical outcomes. Through a multidisciplinary approach encompassing lifestyle modifications, pharmacotherapy, invasive interventions, and treatment of underlying conditions, clinicians can effectively alleviate symptoms, mitigate arrhythmia burden, and improve the overall quality of life for individuals affected by PVCs at rest.