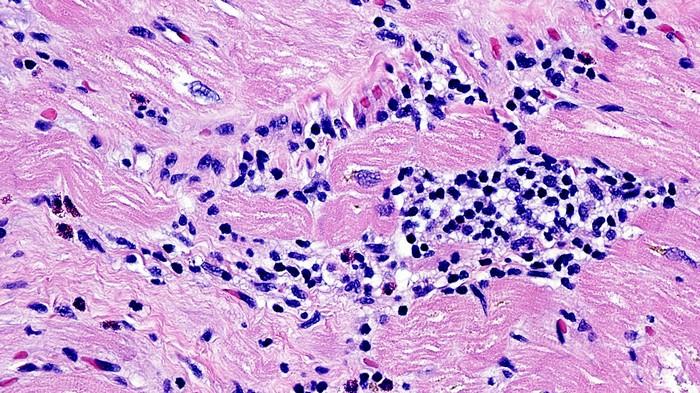
Giant Cell Myocarditis (GCM) stands as a rare and enigmatic cardiac disorder, characterized by inflammation within the heart muscle. This condition poses significant challenges in diagnosis and treatment, often leading to severe cardiac complications and even mortality if left untreated. While much progress has been made in understanding the pathophysiology of GCM, its exact causes remain elusive. In this article, we delve into the intricate mechanisms underlying GCM and explore the factors that contribute to its onset and progression.
1. Overview of Giant Cell Myocarditis
GCM falls under the umbrella of myocarditis, a broader category encompassing various inflammatory conditions affecting the myocardium, or heart muscle. Unlike other forms of myocarditis, GCM is distinguished by the presence of multinucleated giant cells within the myocardial tissue, along with extensive inflammation and damage to cardiac cells.
The clinical presentation of GCM can vary widely, ranging from mild symptoms such as fatigue and chest pain to more severe manifestations like heart failure, arrhythmias, and sudden cardiac death. Due to its rarity and diverse clinical picture, GCM often poses diagnostic challenges, requiring a multidisciplinary approach involving cardiology, pathology, and immunology specialists.
2. Immunological Factors
Immunological dysregulation is thought to play a central role in the development of GCM. The immune system, designed to protect the body from foreign invaders, can sometimes malfunction and mistakenly target healthy tissues, leading to autoimmune diseases such as GCM.
One proposed mechanism involves the activation of T lymphocytes, a type of immune cell, in response to unknown triggers within the myocardium. These activated T cells then recruit other immune cells, including macrophages, which contribute to tissue inflammation and the formation of giant cells.
Furthermore, genetic predispositions may influence an individual’s susceptibility to autoimmune responses, with certain genetic markers being associated with an increased risk of developing GCM. Studies have also suggested a potential link between GCM and viral infections, wherein viral antigens may trigger an immune response that inadvertently targets cardiac tissues.
3. Viral Triggers and Infections
Viruses have long been implicated in the pathogenesis of myocarditis, including GCM. Enteroviruses, adenoviruses, and parvovirus B19 are among the viruses commonly associated with myocarditis, although their precise role in GCM remains incompletely understood.
Viral myocarditis typically occurs when a viral infection spreads to the heart, leading to an inflammatory response. In some cases, the immune system’s attempt to clear the virus may result in collateral damage to cardiac cells, contributing to the development of GCM.
While viral triggers are suspected in many cases of GCM, not all patients with GCM have a clear history of viral infection. This suggests that other factors, such as genetic predispositions or environmental triggers, may interact with viral exposure to precipitate GCM development.
4. Environmental and Toxic Exposures
Environmental factors and toxic exposures have also been proposed as potential triggers for GCM. These may include exposure to certain chemicals, pollutants, or medications that can elicit an immune response or directly damage cardiac tissues.
For example, some studies have explored the role of heavy metals such as lead and mercury in myocardial inflammation and autoimmune reactions. Additionally, certain medications, particularly those with known cardiotoxic effects, may increase the risk of developing myocarditis and GCM in susceptible individuals.
Other environmental factors, such as exposure to mold, allergens, or inflammatory stimuli, could also contribute to the immune dysregulation observed in GCM patients. Further research is needed to elucidate the specific environmental triggers and their mechanisms of action in GCM pathogenesis.
5. Autoimmune Mechanisms
Autoimmune processes are at the core of GCM pathophysiology, wherein the immune system mistakenly targets self-antigens within the myocardium. This results in a cascade of inflammatory responses, tissue damage, and the formation of giant cells characteristic of GCM.
Several autoimmune diseases have been associated with an increased risk of developing GCM, highlighting the interconnectedness of autoimmune pathways. Rheumatoid arthritis, systemic lupus erythematosus (SLE), and autoimmune thyroid disorders are among the conditions that may predispose individuals to GCM.
Autoantibodies, which are antibodies that target the body’s own tissues, have also been detected in GCM patients. These autoantibodies may contribute to the ongoing immune-mediated damage seen in GCM, perpetuating a cycle of inflammation and cardiac dysfunction.
6. Genetic Susceptibility
Genetic factors play a significant role in determining an individual’s susceptibility to autoimmune diseases and myocarditis, including GCM. Certain genetic variations have been identified as potential risk factors for GCM development, although the precise genetic mechanisms remain under investigation.
Genome-wide association studies (GWAS) have identified specific gene polymorphisms associated with increased susceptibility to myocarditis and GCM. These genes are often involved in immune regulation, antigen presentation, and inflammatory pathways, highlighting the complex interplay between genetics and immune responses in GCM pathogenesis.
Family history may also be a relevant factor, as individuals with relatives affected by autoimmune diseases or myocarditis may have a higher likelihood of developing GCM. However, the inheritance patterns and genetic markers involved in familial GCM cases are not fully understood and require further study.
7. Treatment Implications
Understanding the diverse causes and underlying mechanisms of GCM is crucial for developing targeted treatment strategies. Current management approaches often involve immunosuppressive therapies aimed at modulating the immune response and reducing inflammation within the myocardium.
Corticosteroids, such as prednisone, are commonly used as first-line agents to suppress immune activity and mitigate cardiac inflammation in GCM patients. Additional immunosuppressive medications, such as azathioprine, mycophenolate mofetil, and cyclosporine, may be prescribed in refractory cases or to reduce corticosteroid dependence.
In severe cases of GCM with progressive heart failure or arrhythmias, heart transplantation may be considered as a life-saving intervention. However, the success of transplantation in GCM patients depends on various factors, including disease severity, overall health status, and availability of donor organs.
8. Future Directions
Continued research into the etiology and pathophysiology of GCM is essential for advancing our understanding of this complex disorder. Emerging technologies, such as single-cell sequencing and advanced imaging modalities, offer new insights into immune cell populations, molecular pathways, and tissue interactions in GCM.
Precision medicine approaches, including biomarker discovery and personalized immunotherapy, hold promise for tailoring treatment strategies to individual patients based on their unique immune profiles and genetic backgrounds. Collaborative efforts among researchers, clinicians, and patients are needed to drive progress in GCM research and improve patient outcomes.
In conclusion, Giant Cell Myocarditis represents a challenging clinical entity with multifaceted etiological factors, including immunological dysregulation, viral triggers, environmental exposures, autoimmune mechanisms, and genetic susceptibility. By unraveling the mysteries surrounding GCM causes and pathogenesis, we can pave the way for more effective diagnostic approaches and targeted therapies, ultimately improving the prognosis for patients affected by this rare cardiac condition.