Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide. Understanding the pathogenesis of CAD is essential for developing effective prevention and treatment strategies. This article will delve into the mechanisms and factors involved in the development of CAD, covering the roles of endothelial dysfunction, lipid accumulation, inflammation, and genetic predispositions.
Introduction
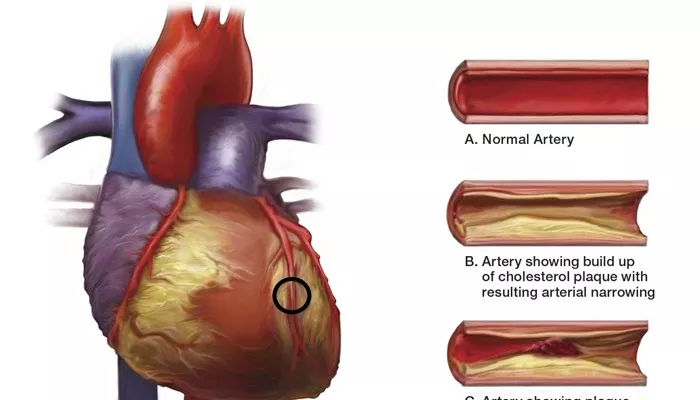
Coronary artery disease (CAD) refers to the narrowing or blockage of the coronary arteries, primarily due to atherosclerosis.
This condition can lead to reduced blood flow to the heart muscle, resulting in chest pain (angina), heart attacks (myocardial infarctions), and other serious cardiovascular events. The pathogenesis of CAD is complex and multifactorial, involving a combination of genetic, environmental, and lifestyle factors.
What Is The Pathogenesis of Coronary Artery Disease
1. Endothelial Dysfunction
Endothelial dysfunction is one of the earliest events in the pathogenesis of CAD. The endothelium, a thin layer of cells lining the blood vessels, plays a crucial role in maintaining vascular homeostasis. It regulates vascular tone, blood flow, and inflammation, and serves as a barrier between the blood and the vessel wall.
Causes of Endothelial Dysfunction
Hypertension: High blood pressure can damage the endothelial cells, making them more susceptible to atherosclerosis.
Smoking: Tobacco smoke contains chemicals that can harm the endothelium, promoting inflammation and oxidative stress.
Diabetes: High blood sugar levels can lead to the formation of advanced glycation end-products (AGEs), which damage the endothelium.
Hyperlipidemia: Elevated levels of low-density lipoprotein (LDL) cholesterol can penetrate the endothelial layer and become oxidized, triggering inflammatory responses.
see also: 7 Clinical Manifestations of Congenital Heart Disease
2. Lipid Accumulation and Plaque Formation
The accumulation of lipids, particularly oxidized LDL (oxLDL), in the arterial wall is a hallmark of atherosclerosis. This process begins when LDL particles penetrate the endothelium and become trapped in the subendothelial space.
Steps in Lipid Accumulation
LDL Oxidation: Once inside the arterial wall, LDL particles undergo oxidation, forming oxLDL, which is highly atherogenic.
Macrophage Recruitment: OxLDL attracts monocytes from the bloodstream, which differentiate into macrophages upon entering the arterial wall.
Foam Cell Formation: Macrophages engulf oxLDL through scavenger receptors, transforming into foam cells. These foam cells accumulate to form fatty streaks, the earliest visible lesions in atherosclerosis.
Plaque Development: Over time, fatty streaks evolve into more complex atherosclerotic plaques, consisting of a lipid-rich core, necrotic tissue, and a fibrous cap.
3. Inflammation and Immune Response
Inflammation is a critical component of CAD pathogenesis. It is both a response to and a driver of atherosclerosis. Various immune cells and cytokines play significant roles in the inflammatory process within the arterial wall.
Key Players in Inflammation
Cytokines: Pro-inflammatory cytokines like interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) are released by endothelial cells, macrophages, and other immune cells, promoting further recruitment and activation of immune cells.
T-cells: These immune cells contribute to the inflammatory milieu by releasing cytokines and interacting with other cells within the plaque.
Mast Cells: These cells release proteolytic enzymes and cytokines that can destabilize the fibrous cap, increasing the risk of plaque rupture.
4. Role of Smooth Muscle Cells
Smooth muscle cells (SMCs) also play a vital role in the pathogenesis of CAD. They contribute to plaque stability and composition through several mechanisms.
SMC Functions in Atherosclerosis
Migration and Proliferation: In response to inflammatory signals and growth factors, SMCs migrate from the media to the intima of the arterial wall and proliferate, contributing to the fibrous cap formation.
Extracellular Matrix Production: SMCs produce extracellular matrix components like collagen and elastin, which provide structural support to the plaque.
Phenotypic Switching: SMCs can switch from a contractile to a synthetic phenotype, enhancing their ability to produce extracellular matrix and contribute to plaque stability.
5. Plaque Rupture and Thrombosis
The ultimate clinical manifestation of CAD often involves plaque rupture and subsequent thrombosis. Plaque rupture exposes the lipid-rich core to the bloodstream, triggering the formation of a blood clot (thrombus) that can occlude the coronary artery, leading to a heart attack.
Factors Influencing Plaque Stability
Fibrous Cap Integrity: A thin, weakened fibrous cap is more prone to rupture. Factors like inflammation, matrix metalloproteinases (MMPs), and proteolytic enzymes can degrade the cap.
Inflammatory Activity: High levels of inflammatory activity within the plaque increase the likelihood of rupture.
Mechanical Stress: Hemodynamic forces such as shear stress can influence plaque stability and contribute to rupture.
Genetic and Epigenetic Factors
Genetic predisposition plays a significant role in the development of CAD. Numerous genetic loci have been identified that influence susceptibility to atherosclerosis and CAD.
Genetic Contributions
Familial Hypercholesterolemia: Mutations in genes like LDLR, APOB, and PCSK9 can lead to significantly elevated LDL cholesterol levels and early onset of CAD.
Polymorphisms: Single nucleotide polymorphisms (SNPs) in genes involved in lipid metabolism, inflammation, and endothelial function can modulate CAD risk.
Epigenetic Influences
Epigenetic modifications, such as DNA methylation, histone modification, and non-coding RNA regulation, also play a role in CAD.
These modifications can alter gene expression in response to environmental factors like diet, smoking, and stress.
Environmental and Lifestyle Factors
While genetic factors are important, environmental and lifestyle factors are critical in the pathogenesis of CAD. These factors can interact with genetic predispositions to influence CAD development.
Key Environmental and Lifestyle Factors
Diet: Diets high in saturated fats, trans fats, and cholesterol can promote atherosclerosis. Conversely, diets rich in fruits, vegetables, whole grains, and healthy fats can reduce CAD risk.
Physical Activity: Regular physical activity helps maintain healthy blood pressure, cholesterol levels, and weight, reducing CAD risk.
Smoking: Smoking is a major risk factor for CAD, as it promotes endothelial dysfunction, inflammation, and oxidative stress.
Stress: Chronic stress can contribute to hypertension and other risk factors for CAD.
Conclusion
The pathogenesis of coronary artery disease is a multifactorial process involving endothelial dysfunction, lipid accumulation, inflammation, smooth muscle cell activity, genetic predisposition, and environmental factors. Understanding these mechanisms provides insight into potential therapeutic targets and strategies for preventing and managing CAD. By addressing the modifiable risk factors and understanding the underlying mechanisms, healthcare providers can better tailor prevention and treatment strategies to reduce the burden of CAD.