The long QT interval, a crucial aspect of cardiac electrophysiology, represents a key marker for understanding arrhythmias.
This article delves into the mechanisms by which a prolonged QT interval leads to arrhythmias, providing a comprehensive overview of its implications for patient management and clinical practice. By exploring the underlying pathophysiology, clinical manifestations, and management strategies, we aim to illuminate the complex relationship between long QT syndrome (LQTS) and arrhythmias.
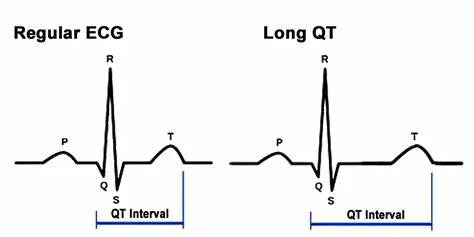
Introduction to QT Interval And Its Significance
The QT interval, measured on an electrocardiogram (ECG), reflects the time taken for the ventricles of the heart to depolarize and then repolarize. It begins at the onset of the Q wave and ends at the end of the T wave. This interval is crucial because it indicates the duration of ventricular electrical activity. A prolonged QT interval signifies an abnormal delay in ventricular repolarization, which can predispose individuals to life-threatening arrhythmias.
Mechanisms Leading to Prolongation of the QT Interval
Several factors contribute to the prolongation of the QT interval, categorized broadly into genetic and acquired causes.
1. Genetic Causes
Genetic mutations play a significant role in congenital long QT syndrome (LQTS), where specific ion channel abnormalities lead to delayed repolarization.
LQT1 (Congenital Long QT Syndrome Type 1): Caused by mutations in the KCNQ1 gene, which encodes for the potassium ion channel responsible for the I_Ks current. This current is crucial for the rapid repolarization of the ventricles.
Defects in this channel lead to a prolonged action potential duration and increased risk of arrhythmias.
LQT2 (Congenital Long QT Syndrome Type 2): Associated with mutations in the KCNH2 gene, also affecting the I_Kr current, another potassium ion channel crucial for ventricular repolarization. These mutations similarly disrupt the normal repolarization process.
LQT3 (Congenital Long QT Syndrome Type 3): Caused by mutations in the SCN5A gene, which encodes the cardiac sodium channel. This mutation affects the cardiac action potential’s depolarization phase and can prolong the QT interval indirectly by altering the balance of ion currents during repolarization.
SEE ALSO: What Are The Treatments for Atrial Arrhythmia?
2. Acquired Causes
Acquired long QT syndrome (aLQTS) can arise from various external factors, including:
Medications: Several drugs are known to induce QT prolongation as a side effect. Examples include antiarrhythmics (e.g., sotalol, dofetilide), antipsychotics (e.g., haloperidol), and antibiotics (e.g., erythromycin). These drugs may interfere with ion channel function or affect the cardiac repolarization process.
Electrolyte Imbalances: Abnormal levels of potassium, calcium, or magnesium can disrupt normal cardiac repolarization.
Hypokalemia and hypomagnesemia are particularly noted for their role in QT prolongation.
Structural Heart Disease: Conditions such as cardiomyopathy or myocardial infarction can alter the electrical properties of the heart, contributing to QT interval prolongation.
How Long QT Interval Causes Arrhythmias
The prolonged QT interval increases the risk of arrhythmias primarily through mechanisms involving abnormal repolarization and altered cardiac electrophysiology.
1. Early Afterdepolarizations (EADs)
One of the primary arrhythmogenic mechanisms associated with a prolonged QT interval is the development of early afterdepolarizations (EADs). EADs occur during the repolarization phase of the cardiac action potential, when the cell membrane potential becomes unstable. This instability can lead to premature and inappropriate activation of cardiac cells, potentially initiating reentrant arrhythmias.
Mechanism: EADs are often triggered by prolonged cardiac repolarization due to impaired ion channel function. In a prolonged QT scenario, the repolarization phase is extended, making the cardiac cells more susceptible to premature depolarizations.
Implications: EADs are associated with increased susceptibility to ventricular tachycardia and ventricular fibrillation, both of which can result in sudden cardiac death if not promptly managed.
2. Torsades de Pointes (TdP)
Torsades de Pointes (TdP) is a specific type of polymorphic ventricular tachycardia closely associated with a prolonged QT interval. It is characterized by a distinctive twisting pattern on the ECG.
Mechanism: TdP often results from EADs that lead to triggered activity. The prolonged QT interval creates a substrate where EADs can initiate TdP, particularly in the setting of increased sympathetic tone or electrolyte imbalances.
Clinical Relevance: TdP can be self-limiting but may also progress to more severe arrhythmias. It is crucial to identify and manage the underlying cause of QT prolongation to prevent TdP episodes.
3. Reentrant Arrhythmias
Prolonged QT intervals can also facilitate reentrant arrhythmias due to altered conduction properties.
Mechanism: The dispersion of repolarization times across different regions of the heart can create areas of slower conduction. These areas may serve as substrates for reentrant circuits, leading to sustained arrhythmias.
Clinical Impact: Reentrant arrhythmias can result in persistent tachycardia, which can impair cardiac function and increase the risk of sudden cardiac death.
Management Strategies
Effective management of long QT interval involves addressing both the underlying causes and the risk of arrhythmias.
1. Lifestyle and Medication Adjustments
Drug Avoidance: Discontinuing or replacing medications that may prolong the QT interval.
Electrolyte Management: Correcting any imbalances in potassium, calcium, or magnesium levels.
2. Specific Treatments for Congenital LQTS
Beta-Blockers: For congenital LQTS, particularly LQT1 and LQT2, beta-blockers are a cornerstone of treatment. They reduce the incidence of arrhythmias by stabilizing cardiac repolarization.
Implantable Cardioverter-Defibrillators (ICDs): In high-risk patients, ICDs may be recommended to prevent sudden cardiac death.
Gene-Specific Therapies: Emerging therapies targeting specific genetic mutations are under investigation and may offer personalized treatment options in the future.
3. Management of TdP
Acute Treatment: Immediate treatment of TdP may include intravenous magnesium and addressing any potential triggers.
Long-Term Prevention: Avoiding known triggers and managing underlying conditions to prevent recurrence.
Conclusion
The long QT interval is a significant marker of arrhythmia risk, with its effects mediated through mechanisms such as early afterdepolarizations, torsades de pointes, and reentrant circuits. Understanding the underlying causes, diagnostic strategies, and management options is crucial for effective patient care. Ongoing research and advances in genetic testing and targeted therapies hold promise for improved outcomes and personalized treatment approaches for patients with long QT syndrome. By recognizing the multifaceted nature of long QT interval and its arrhythmogenic potential, clinicians can better manage and mitigate the risks associated with this condition, ultimately enhancing patient safety and outcomes.